Introduction
The fluid expansion in the bulb of a thermometer would naturally undergo a three-dimensional volumetric change which is a quadratic function. The graduated thermometer stem is a linear device. Is the temperature reading a valid representation of the physics upon which the thermometer is based?
The answer to this, and many other questions, lies in revolutionary discoveries by Howard Lawrence Schriefer in pursuit of a Unified Theory of the universe.
Thermometer Paradox
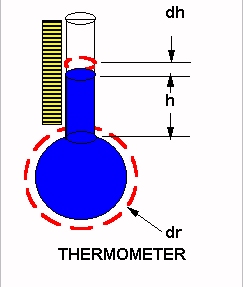
If the fluid in the thermometer bulb were unconstrained and free in space, its spherical volumetric change would be the derivative of the spherical volume with respect to the spherical radius. This derivative is equal to the surface of the sphere, wherein the spherical radius is squared.
The fluid in the stem undergoes its volumetric change as a cylinder of constant cross section; and, the only variable is the linear height. The temperature graduated units on the thermometer stem are equal and linear. The actual expansion of the fluid is a parabolic function, while the actual representation at the scale on the thermometer stem is a straight line. No wonder so many different temperature scales and components thereof are necessary in the sciences of physics, chemistry, heat transfer, and thermodynamics.
The matter of a temperature scale is not simple, as defined by scientists. I'm not talking about contrasts among Fahrenheit, Rankine, Kelvin, and Celsius. There are reliable conversion procedures among these scales which mimic each other. I'm talking about the suitability of a way to measure large temperature differences, so that meaningless statements like "a million degrees" are not used in science. If we wish to have an all encompassing temperature scale, we turn to the Absolute Temperature Scale, which is based upon the Carnot cycle, a reversible (and impossible) thermodynamic process which has no losses. Unfortunately, such a process in reality does not exist. Next best, would be the negotiated International Temperature Scale. This temperature scale uses such reference points as :
-
Temperature of equilibrium between liquid and vapor oxygen @ -182.970° C.
-
Temperature of equilibrium between ice and air-saturated water @ 0.000° C.
-
Temperature of equilibrium between liquid water and steam @ 100.000° C.
-
Temperature of equilibrium between liquid sulfur and its vapor @ 444.000° C.
-
Temperature of equilibrium between solid silver and liquid silver @ 960.800° C.
-
Temperature of equilibrium between solid gold and liquid gold @ 1063.000° C.
Sounds international, doesn't it? Try carrying a set of those in your tool box! It gets even better. This scale requires four different interpolation equations, which Einstein couldn't remember, to find actual temperatures between these points. How do we get to "a million degrees" that many publicized scientists like to throw around?
Seems more logical to just use wavelengths or frequencies in computations rather than go through such gyrations of mathematics. But, that might be too simple. The Schriefer Unified Theory provides for such simplicity.

